CLINICAL DEPARTMENT
...in aesthetic medicine, to ensure the performance and safety of our devices and to support healthcare professionals in the management of patients. We are committed, in collaboration with the research and development teams, to providing effective and safe technologies for the beautification of the face and figure.
MATERIALOVIGILANCE
The collection and analysis of incidents relating to our devices is our priority.
We are committed to doing the necessary follow-up to ensure the safety of our devices.
You are a healthcare professional:
To report an incident or risk of incident relating to a Deleo medical device, please contact the materialovigilance department on 04 94 45 83 75 or at the following address: clinical.research@deleo.fr
CLINICAL INVESTIGATIONS
A high and fast return for your practice.
Our devices are tested to ensure their effectiveness and safety.
DELEO's clinical studies are designed in collaboration with experts in aesthetic medicine and dermatology. They are carried out in centres with volunteer patients, under experimental conditions that meet regulatory requirements.

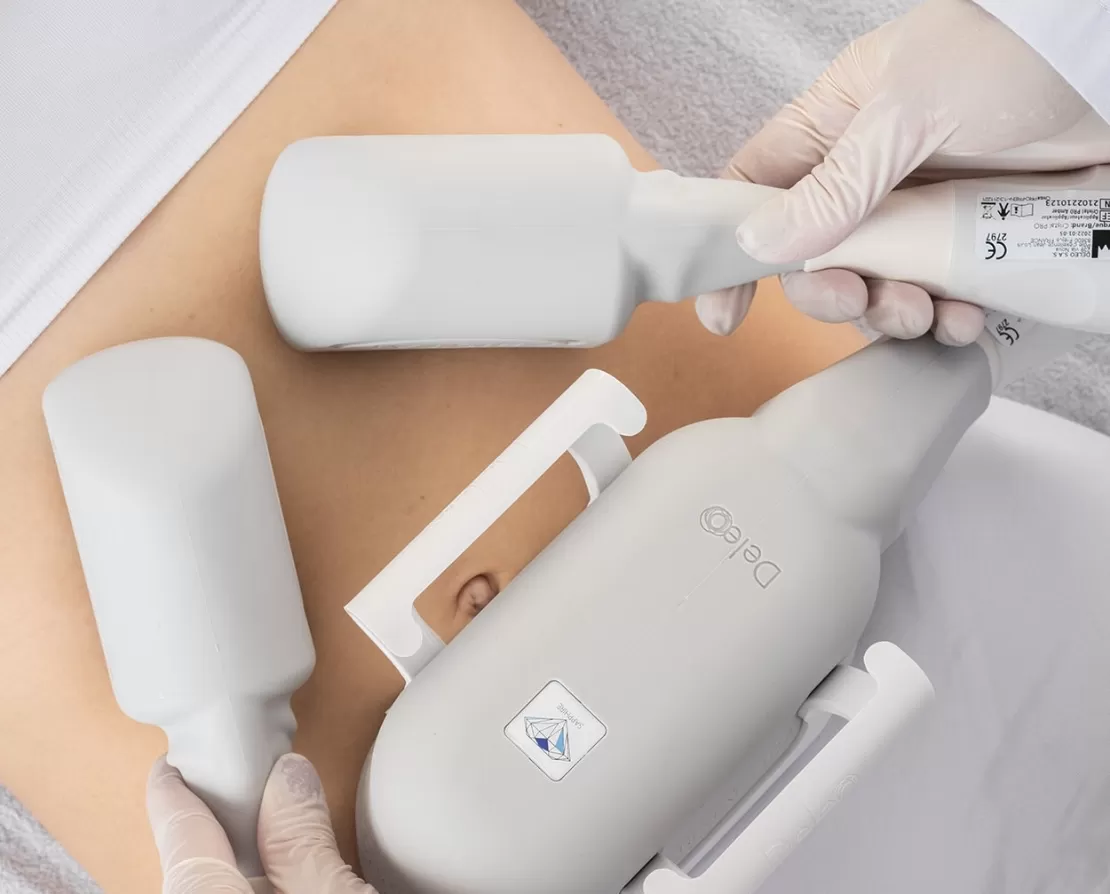
Cryolipolysis studies:
- Interventional study evaluating a cryolipolysis treatment at -12° C for 30 min on the reduction of localized fat clusters on the abdomen and thighs.
- Observational study evaluating CRISTAL PRO cryolipolysis in real conditions of use.
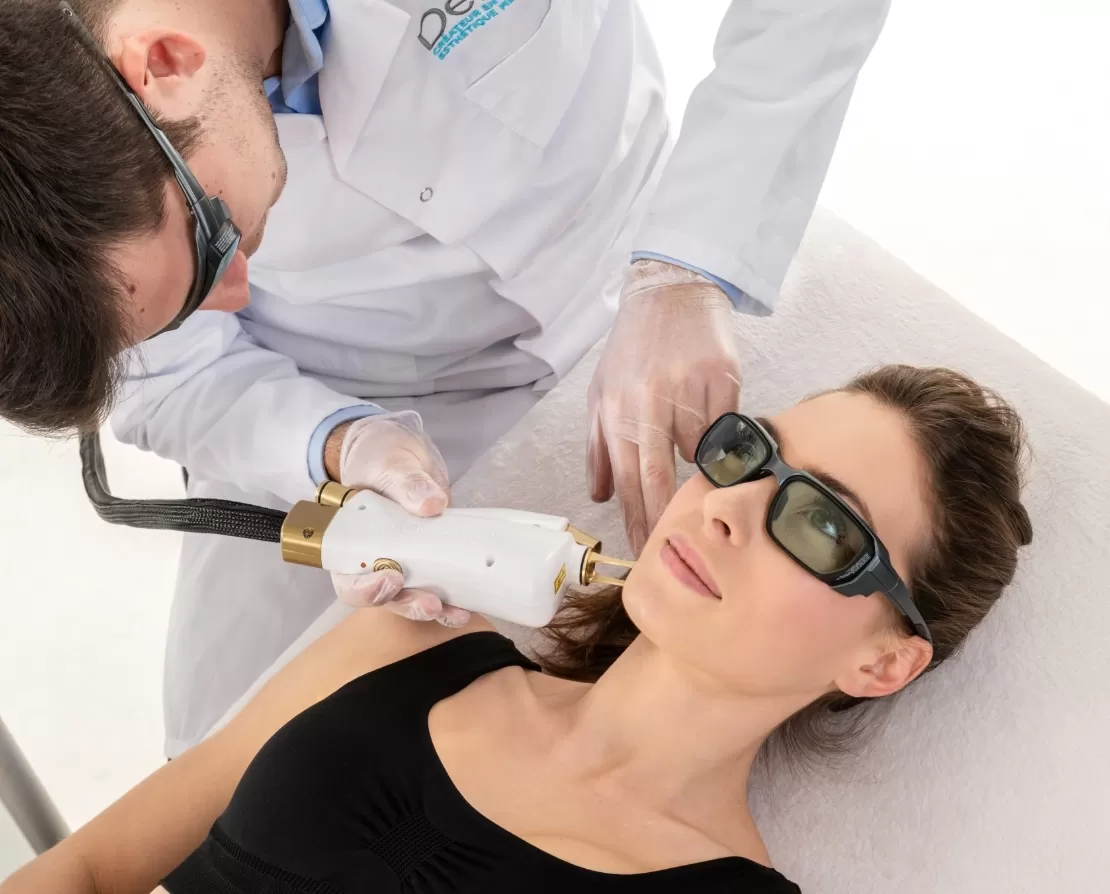
Acne scar study:
Interventional study evaluating one treatment cycle of the Galss ORIGIN Erbium laser on the improvement of acne scars.
Electromagnetic Stimulation Study:
Interventional study evaluating the safety and short-term efficacy of one cycle of electromagnetic stimulation treatment on the improvement of urinary incontinence symptoms in women.